This article referring to CDSCO/RRAS Ver.:00 dated 23/11/2012, talks about the essential components of an effective recall system with the procedure to be followed in India and how Morulaa can help manufacturers and importers manage these challenges in case of any product recall. In the highly regulated healthcare device industry, which is overseen by CDSCO, manufacturers and importers should prioritize patient safety and compliance with regulatory standards. An important aspect of maintaining these standards is the implementation of an efficient recall system for products known or suspected to be defective.
Recall involves the removal of drugs from distribution or use due to reported deficiencies in quality, efficacy, or safety. Quality-related issues may include deviations such as Not of Standard Quality, Adulteration, or Spurious drugs. Safety and efficacy concerns encompass serious adverse reactions and fatalities. Additionally, recalls encompass drugs prohibited under the Provisions of Drugs & Cosmetics Act, as well as products whose licenses are suspended or canceled. The Rapid Alert System, a regulatory tool approved by CDSCO and part of the medical device registration process in India, prioritizes the immediate transmission of alerts for issues deemed urgent and severe. This process often requires updates through the SUGAM portal, ensuring compliance with CDSCO mandates.
Types of Recall
-
Voluntary Recalls: A voluntary recall may be initiated by manufacturers in response to any situation compromising the product’s quality, safety, or efficacy. This can be due to non-adherence to regulatory standards found during post-marketing stability studies, identification of defects from market complaints, failure investigations revealing potential adverse impacts on distributed products (like contamination or mix-ups), unusual findings in retention sample visual inspections indicating quality issues, or post-marketing surveillance and pharmacovigilance reports indicating severe safety risks.
-
Statutory Recall: A statutory recall is launched when Drug Regulatory Authorities, whether at the Central or State level, mandate the withdrawal of products in specific situations. These situations can include recalling drug products or batches that fail to meet legal standards, such as those not of standard quality, withdrawing drugs that have been banned, addressing issues with labeling or promotional materials that violate regulations, or dealing with products that contravene Rule 106 related to Diseases under Schedule J
Recall Classification and Levels: Below are the recall classification and levels issued by the 39th DCC’s guidelines
Recall Classification
-
- Class I recalls involve products that could likely lead to serious health issues or death, including those banned under Section 26A of the Drugs and Cosmetics Act 1940. CDSCO mandates stringent oversight for such products.
-
- Class II recalls are for products that might lead to temporary health issues or have a low chance of causing serious harm. Monitoring the registration updates through SUGAM is important here.
-
- Class III recalls concern products that are unlikely to result in any health problems. The, regulatory compliance with CDSCO guidelines is essential.
Recall Levels
-
- Consumer/User Level: Targets end-users, potentially including individual consumers, patients, doctors, and hospitals, and might cover intermediary wholesale or retail stages.
-
- Retail Level: Involves recalling products one step before reaching the consumer, affecting entities like retail stores, pharmacies, hospital pharmacies, dispensing physicians, and facilities such as clinics and nursing homes.
-
- Wholesale Level: Encompasses all distribution stages from the manufacturer up to the retailer, with SUGAM playing a vital role in communication.
Timelines for Effective Recall System & Rapid Alert
The timeline for implementing a recall system and issuing a rapid alert varies is as below –
-
Class I: 24 to 72 hours
-
Class II: Upto 10 days
-
Class III: Upto 30 days
The recall process begins upon receiving information from either the State or Central Drugs Control Department regarding a statutory recall or when a manufacturer voluntarily initiates a recall. Recalls must start immediately, regardless of the outcomes under Sections 25(3) and 25(4) of the Drugs & Cosmetics Act 1940 concerning the submission of evidence.
Overview of Process Flow Rapid Alert & Recall System
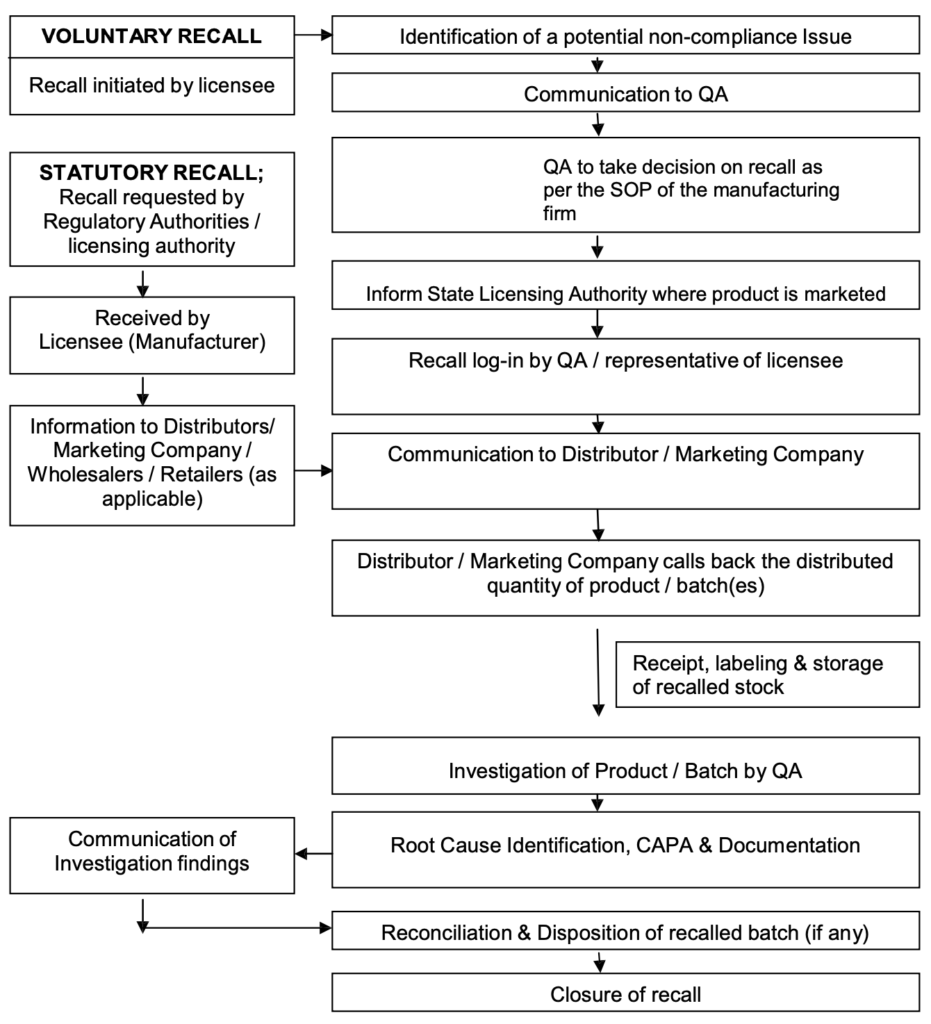
Procedure for Recall
The foundation of a secure and effective recall system lies in the preparedness and organization of the product manufacturers or importers. Key elements of this strategy include:
-
Designation of Responsibility: An authorized individual must be appointed to oversee and coordinate recall efforts. This role involves ensuring that adequate staffing and resources are available to manage recalls efficiently and effectively.
-
Development of Written Procedures: Clear, written procedures for organizing and executing recall activities is important. These procedures should be regularly reviewed and updated, detailing how recalls can be initiated at the necessary level within the distribution chain.
-
Segregation of Recalled Products: Recalled items must be stored in a secure, segregated area to prevent their re-entry into the market.
-
Notification of Licensing Authorities: Regulatory bodies, such as the CDSCO in India, must be informed of any recall plans, highlighting the importance of transparency and communication with licensing authorities.
-
Accessible Distribution Records: Detailed records of distribution, including information on wholesalers and direct customers, must be easily accessible. This ensures that recalls can be carried out effectively, reaching all affected products in the distribution chain.
-
Monitoring and Documentation: The recall process must be closely monitored and documented, including the final disposition of the recalled product. A final report reconciling delivered and recovered product quantities is important for evaluating the recall’s effectiveness.
Follow-Up Action of Recalled Goods
Post-recall actions includes evaluating the recall’s effectiveness, uncovering the cause, and taking steps to prevent similar issues. The recall process is monitored by the licensee or their representative, or the Quality Assurance (QA) Head, to ensure its progress. Recalled products are quarantined and stored securely. The QA Head may also inspect and sample these goods to identify the quality defect’s root cause. An investigation into the recalled batches, guided by the licensee’s standard operating procedures on addressing non-conformities, aims to find the root cause and initiate corrective and preventative actions. An impact assessment is necessary for all batches of the implicated product and may extend to batches of other products if relevant. Should a recall’s cause be linked to a quality issue with any raw material, tracing this material across all affected product batches is important. This involves reviewing transaction records to pinpoint which batches or products incorporated the problematic material. Monitoring key data—material, plant, and batch numbers—is essential for tracking the raw material’s usage across different formulations and understanding its role.
After thorough reconciliation, decisions regarding the recall of affected batches are made based on a product quality assessment. The outcome of the investigation will guide the QA Head or licensee representative in instructing the distributor or marketing company on the appropriate handling of recalled goods, adhering to regulatory standards.
Conclusion
For healthcare manufacturers and importers, establishing a system for the prompt and effective recall of defective products is not just a regulatory requirement—it’s an essential component of patient safety and brand integrity. By incorporating the key elements outlined above and partnering with Morulaa, companies can ensure they are well-prepared to manage recalls effectively, maintaining compliance with CDSCO regulations and safeguarding public health. This is crucial for any organization operating in the medical device sector in India, where stringent registration and regulatory standards governed by CDSCO and facilitated through tools like the SUGAM portal and MD-14 forms are the norm. To ensure you are maintaining and following compliances for your products in India, email us now on [email protected] or Click Here
