Medical Device Amendments – Interventional Radiology
Introduction The Central Drugs Standard Control Organization (CDSCO) is responsible for the registration of medical devices and ensuring compliance with Indian medical device regulations. The Drugs Controller General of India (DCGI) oversees product approvals, regulates medical devices, and ensures the quality of imported devices. CDSCO also coordinates with State Drug Control Organizations to maintain uniform […]
Regulatory Consultancy for Medical Device Amendments in Cardiovascular Devices
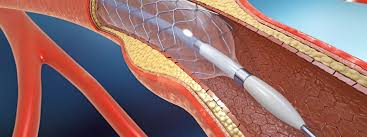
Introduction The classification and approval of cardiovascular medical devices regulations in India are governed by the Medical Device Rules 2017, under the Central Drugs Standard Control Organization (CDSCO). CDSCO categorizes these devices into Class A, B, C, and D based on risk level, from low-risk products like pacemaker test magnets to high-risk devices like implantable […]
