Indian MDR Amendments in Dermatological and Plastic Surgery

Indian MDR Amendments in Dermatological and Plastic Surgery India’s medical device regulatory framework continues to evolve, impacting various medical specialties, including dermatology and plastic surgery. The Central Drugs Standard Control Organization (CDSCO) oversees the medical device regulatory requirements in India, ensuring that all devices comply with safety and efficacy standards. Under the Drugs and Cosmetics […]
Finding the Right Medical Device Distributor in India

Introduction The Indian medical device market is growing rapidly and is expected to reach $50 billion by the end of 2025. This creates a huge opportunity not just for medical device manufacturers but for distributors as well. Being successful in India is not only about getting your product approved, but also finding the right distributor […]
Medical Device Amendments for Devices Used in Physical Support
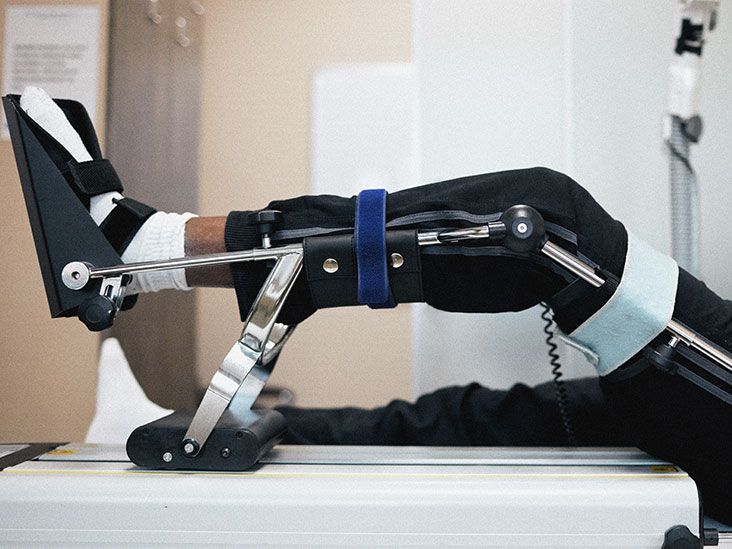
CDSCO Medical Device Registration: Amendments for Devices Used in Physical Support This article covers recent CDSCO medical device registration amendments related to the intended use of medical devices in the physical support category. The Central Drugs Standard Control Organization (CDSCO) is India’s regulatory authority overseeing pharmaceuticals and medical device regulations. All notified medical products, including […]
