India Risk Classification for Physical Support as Medical Device

This article deals with the latest rules of Physical Support as a Medical Device in India – Non Notified Medical Device Registration. Indian healthcare regulators at the Central Drugs Standard Control Organization (CDSCO) have issued substantial new risk-based classification lists for Physical Support and Medical Device in India. All Physical Support Products now need to […]
India Risk Classification for Rehabilitation as Medical Device
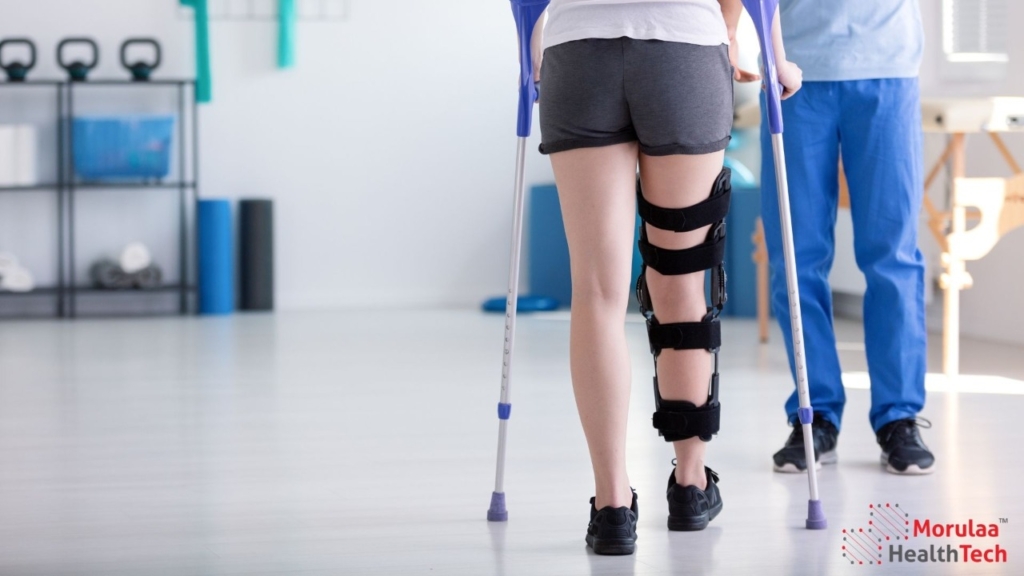
This article deals with the latest rules of Rehabilitation Medical Device in India –Non Notified Medical Device Registration. Indian healthcare regulators at the Central Drugs Standard Control Organization (CDSCO) have issued substantial new risk-based classification lists for Rehabilitation and Medical Device in India. All Rehabilitation Products now need to be registered in order to comply […]
India Risk Classification for Ophthalmology as Medical Device

This article deals with the latest rules of Ophthalmology as a Medical Device in India – Non Notified Medical Device Registration. Indian healthcare regulators at the Central Drugs Standard Control Organization (CDSCO) have issued substantial new risk-based classification lists for Ophthalmology as Medical Device in India. All Ophthalmology Products now need to be registered in […]
India Risk Classification for Obstetrical and Gynecological as Medical Device
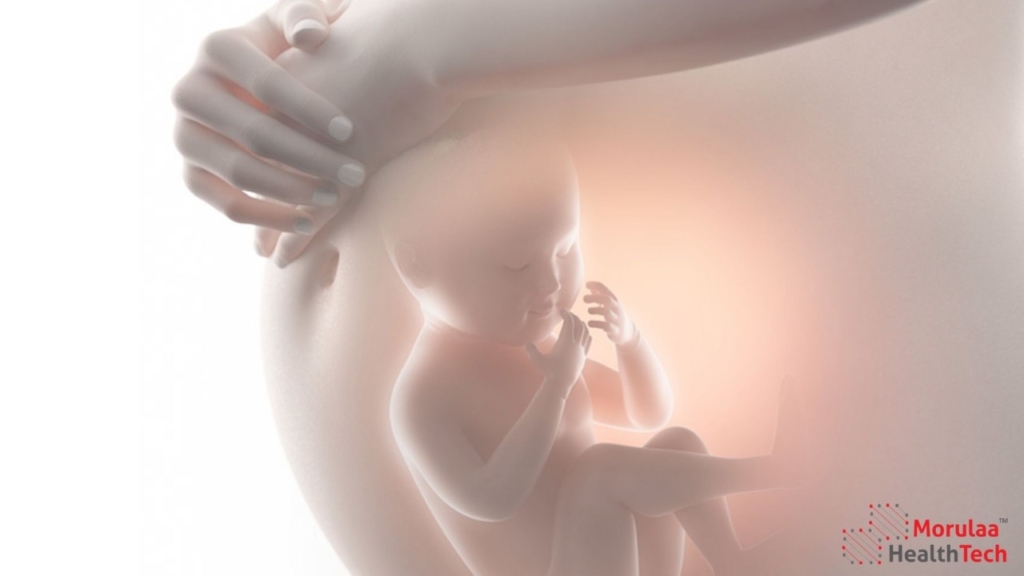
Introduction – Classification of Obstetrical and Gynecological Products as per Medical Device Rules 2017 in India The Medical Device Rules 2017, implemented by the Central Drugs Standard Control Organization (CDSCO), serve as the regulatory framework for medical devices in India. Under these rules, obstetrical and gynecological products are classified into different risk categories to ensure […]
