India Risk Classification for Gastroenterology as Medical Device
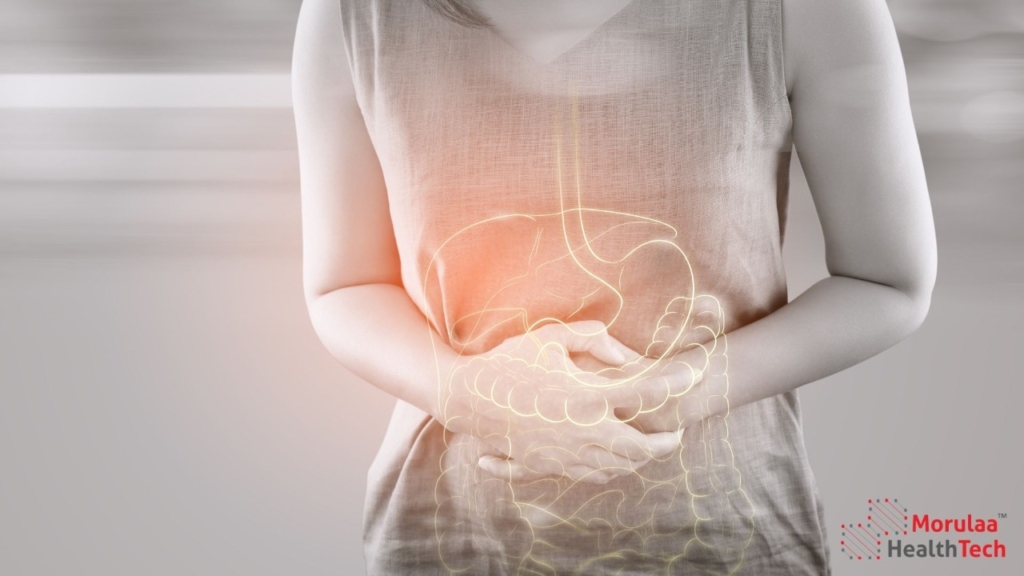
Introduction – Gastroenterology as Medical Device India Need Help with Gastroenterology Device Registration? Contact Us India is Updating Existing Risk-Based Classifications for Gastroenterology Products As per the latest notification MED-16014(12)/1/2024-eoffice released on January 6th, 2025, 10 devices in this category have been reclassified from Class A to Class A (Self Notified). This change simplifies compliance […]
India Risk Classification for ENT as Medical Device

Introduction: CDSCO guidelines for ENT devices This article focuses on the classification and regulation of ENT as a Medical Device under the Medical Device Rules 2017 ENT devices in India. The Central Drugs Standard Control Organization (CDSCO) has established specific guidelines for medical devices, including Class A devices, which are considered low-risk and classified as […]
India Risk Classification for Dental as Medical Device
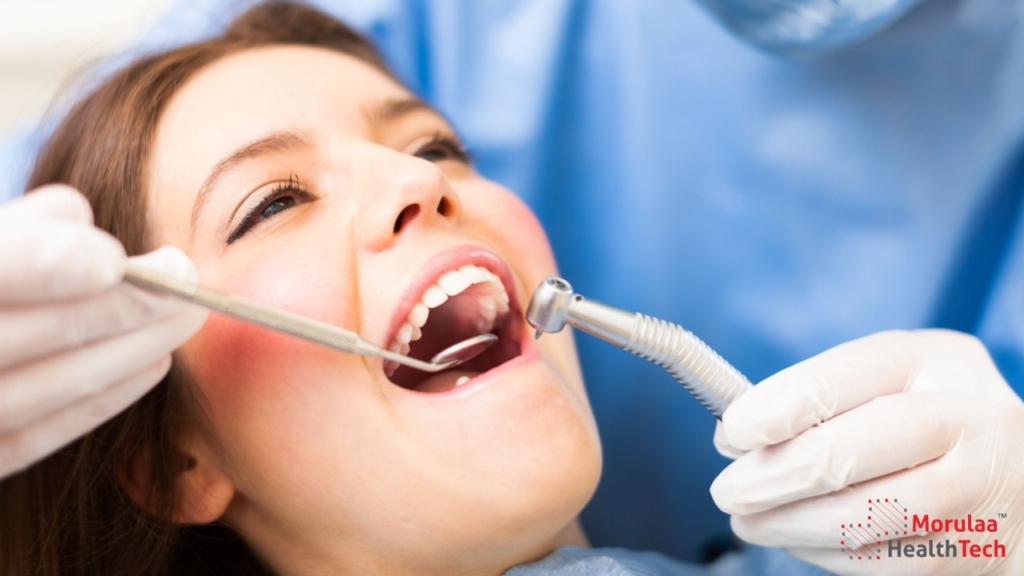
Introduction: CDSCO guidelines for dental medical devices This article focuses on the classification and regulation of dental devices as Medical Devices under the Medical Device Rules 2017 dental devices in India. The Central Drugs Standard Control Organization (CDSCO) has established specific guidelines for medical devices, including Class A and Class B devices, which range from […]
India Risk Classification for Cardiovascular as Medical Device
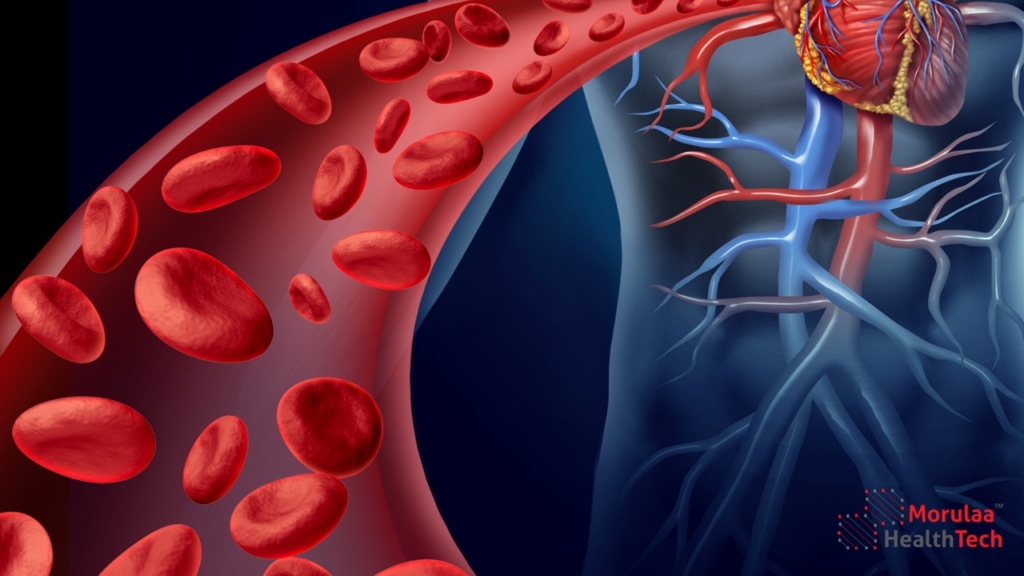
Introduction This article provides an overview of the classification and regulation of cardiovascular medical devices in India under the Medical Device Rules 2017. The Central Drugs Standard Control Organization (CDSCO) has established guidelines for regulatory compliance for cardiovascular devices, categorizing them into Class A, B, C, and D based on risk levels. These regulations ensure […]
India Risk Classification for Pain Management as Medical Device
Introduction – Pain Management as Medical Device India This article focuses on pain management medical devices regulatory aspects and the classification of pain management devices as Medical Devices under the Medical Device Rules 2017 in India. The Central Drugs Standard Control Organization (CDSCO) has established specific guidelines for medical device classification India, categorizing them into […]
India Risk Classification for Anesthesiology as Medical Device
Introduction: CDSCO Anesthesia Device Registration and Classification in India This article focuses on Medical Device Registration Anesthesiology India and the regulatory classification of anesthesiology medical devices under the Medical Device Rules 2017 in India. The Central Drugs Standard Control Organization (CDSCO) has provided specific guidelines for the classification of anesthesiology devices, categorizing them into Class […]
Medical Devices Amendments Given By CDSCO
This article deals with various amendments which happened in the fields of Anesthesiology, Cardiovascular, Physical Support, Radiology, Dermatological & Plastic Surgery and Rehabilitation. With several changes being constantly introduced by the CDSCO, this current change is mainly dealing with the Intended use of Various Products. The CDSCO has classified the Medical Device Registration Appendix A […]
