Notified Medical Devices India

A. Background: Apply for MD- 15 License on SUGAM India Medical Device Regulations The recent political changes resulting in a pro-business environment having facilitated medical device approval in India, drawing significant attention from the Healthcare industry to stand up and take notice. In 2023, the Indian healthcare industry reached a value of US$ 372 billion […]
Statistics & Figures: Medical Device Registration India
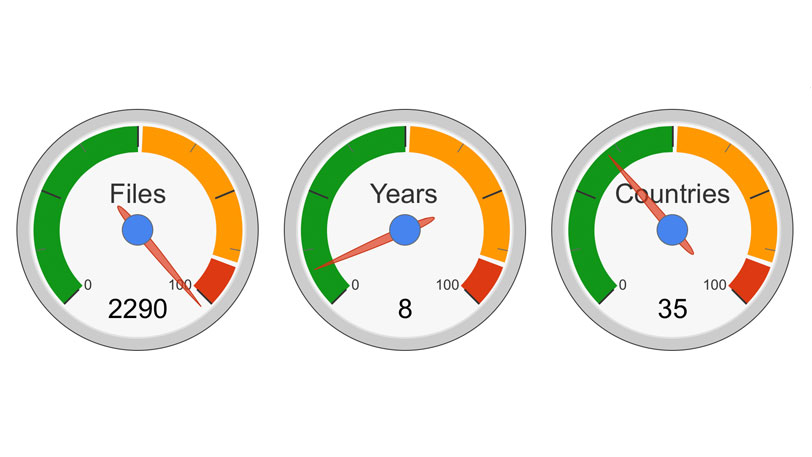
The requirements for medical device registration in India with the CDSCO is explained in this blog. In brief, the CDSCO came into existence in 2006 and has since then implanted several rules and regulations for medical device imports into the country. Medical devices which have approval from GHTF countries can register in India through a straight forward document driven process. Medical […]
